
And yet there’s another electron in the second shell, shell number two. But in this particular scenario, it’s only holding one electron. Let’s recall that the very first shell, the shell nearest to the nucleus, which we will label as shell number one, can hold up to two electrons. To understand what we mean by excited state, we need to first recall that each electron energy level or shell can only hold up to a certain number of electrons. So anyway, what we’ve been asked to do is to find how many of the electrons in the atom are in excited states. There are one, two electrons actually shown in the diagram. And all this is telling us then is that there are two electrons in this atom.īut then we can see this from the diagram already. Therefore, the number of positive charges must balance the number of negative charges in order for the atom to be electrically neutral. And, the reason for this is that protons are positively charged and electrons are negatively charged. Now, the fact that this atom is electrically neutral means that the number of electrons in this atom must be the same as the number of protons in the nucleus of this atom.
HELIUM PROTONS NEUTRONS ELECTRONS PLUS
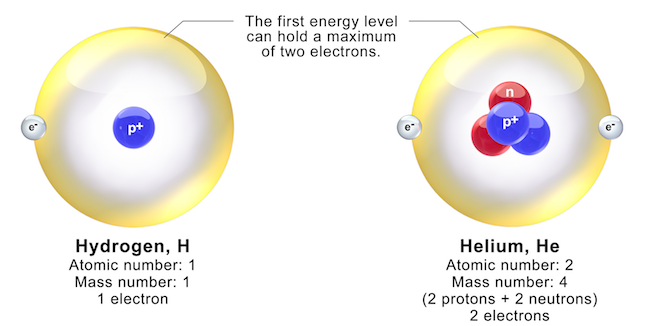
And therefore, we know that there must be two protons in the nucleus, plus however many neutrons, which is not relevant to us right now. If we look on the periodic table, we can see that a helium atom is defined as having two protons in its nucleus. Now, we’ve been told in the question that this atom is a helium atom and that it’s electrically neutral. Now, these electrons are occupying these energy levels. And, the blue blobs actually represent electrons themselves. Additionally, we see two black circles which represent the electron energy levels in this atom. And, we can see that this pink blob in the middle must represent the nucleus of the helium atom, which contains all the protons and neutrons in this atom. Okay so, in this diagram we’ve been told that we’ve got a helium atom. How many of the electrons in the atom are in excited states? 1e = 1.602 x 10 -19 Coulombs, so the charge of a hydrogen nucleus is +1e and the charge of a helium nucleus is +2e.The diagram shows an electrically neutral helium atom. What about an oxygen nucleus? Hint: oxygen is atomic number 8.Īs a note, charge may also be expressed in elementary charge units (e). The total charge of a helium nucleus is 2 x 1.602 x 10 -19 Coulombs = 3.204 x 10 -19 Coulombs.

Atomic number 2 is helium, with a nucleus containing two protons and two neutrons. The total charge of all the protons in the hydrogen nucleus is given by 1 x 1.602 x 10 -19 Coulombs. A hydrogen atom, for example, has atomic number 1, meaning its nucleus contains one proton (sometimes accompanied by a neutron in deuterium, or heavy hydrogen, but neutrons do not carry a charge-they just contribute mass), and the atom remains charge neutral when a single electron orbits the nucleus. In fact, the number of protons in the atomic nucleus defines the type of atom. The number of protons contained in the atomic nucleus is different for each chemical element. An atomic nucleus is composed of protons and neutrons, and the nucleus is orbited by electrons.Ī single electron has a charge of -1.602 x 10 -19 Coulombs, a negative charge which exactly cancels the positive charge of one proton. What is the total charge or strength of all the protons in the nucleus of an atom?Ī single proton has a charge of +1.602 x 10 -19 Coulombs.


 0 kommentar(er)
0 kommentar(er)
